Abstract
GMALL Trial 08/2013 (NCT02881086) of the German Multicenter Study Group for Adult ALL (GMALL) yielded promising overall results (Goekbuget et al, ASH 2021). We report herewith detailed and updated results for adult T-ALL/LBL. Patients (pts) aged 18-55 years (yrs) received a 2-phase induction, up to 8 cycles of PEG-asparaginase (ASP) with up to 7 cycles of HDMTX, a reinduction and maintenance up to 2.5 yrs (none in LBL). Standard risk (SR) pts received 2 cycles Nelarabin/Cyclo (1,500 mg/m2 Nela and 200 mg/m2 Cyclo d 1,3,5) as consolidation (C) 3 and 5. CNS prophylaxis included i.th. applications and CNS irradiation (24 Gy). Risk stratification was based on phenotype and MRD (table 1). In high-risk (HR) pts (early/mature T-ALL; thymic T-ALL with late CR or MolFail after C1) allogeneic stem cell transplantation (SCT) was indicated after C1. Pts with MolFail after C1 had one cycle of Nela before SCT. Pts with HR but MolCR after induction were randomized (SCT vs standard therapy; results still blinded). Response assessment included cytology, MRD (IG/TCR PCR), and standard imaging for extramedullary sites. In PR/CRu a PET-scan was recommended after C1.
281 (208 T-ALL; 73 T-LBL) pts with a median age of 30 (18-55) yrs were evaluable (table 1). 46% of T-ALL cases were thymic (CD1a+). The CR/CRu rate was 87% vs 74% in T-ALL vs T-LBL. PET CT was performed in PR (N=14)/CRu (N=14); it was negative in 7 (50%) /12 (86%) resp.. 2/7 pts with PET-positive PR relapsed (1 mediastinum, 1 bone marrow). PR/CRu in conventional imaging did not impact outcome.
MRD markers were not available in 34% of early T-ALL (14%/1% in mature/thymic). MolCR rates differed between T-ALL subtypes (table 1). During induction the overall MolCR rate in relation to all evaluable pts increased from 15% (4%/4%/29%) after induction I to 61% (41%/48%/84%) after induction II and 67% (47%/62%/82%) after C1; the respective numbers for early/mature/thymic subtype are given in brackets (table 1).
14 patients with MolFail (10 earlyT) had Nela after C1 with 1 MolCR (2 were low positive and one negative by flow cytometry). 123 pts with SR T-ALL/LBL had documented Nela during consolidation. The tolerability of C 3 and 5 was good (oIII-IV toxicities ≥=5%: Anemia 7%/3%, lymphopenia 5%/16%, thrombopenia 7%/4%, neutropenia 5%/8%, leukopenia 15%/20%). One pt developed a Guillain-Barré Syndrome post Nela which was reversible.
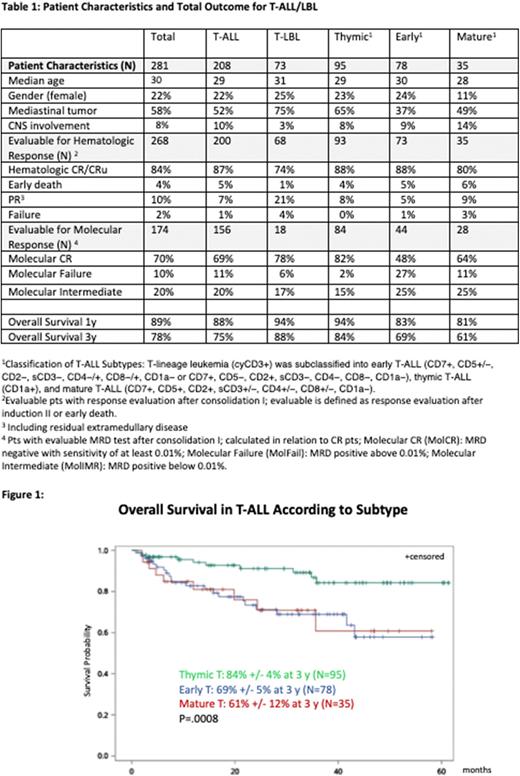
The median follow-up was 28 months (mo). 3y overall survival (OS) for all pts was 78%. 3y OS of T-ALL was 84% for thymic, 69% for early, and 61% for mature T-ALL resp. (p=0.008) (figure 1). Age had no impact on OS in thymic T-ALL (80%, 83%, 95% and 86% for age 18-25, 26-35, 36-45 and 46-55 yrs resp.; P=>0.05), whereas significant differences were observed in early T-ALL (92%, 68%, 56% and 27% resp; p=0.0004). In thymic T-ALL pts significant OS differences were observed for MolCR (N=69), MolFail (N=2) and MolIMR (N=13) (94% vs 50% vs 76% resp. at 3 yrs; P=.0006). 78 HR pts received SCT in CR1 and in these the OS was 68% with CIR and TRM of 26% and 15% at 3 yrs resp.. MRD status after C1 did not significantly impact OS after SCT. Overall, 17 relapses occurred in T-ALL; extramedullary sites were involved in 2 cases (CNS:0). 5 relapses occurred in T-LBL (3 after withdrawal), all with extramedullary involvement (CNS:0).
The results underline that thymic T-ALL (easily identified by CD1a expression) represents a low-risk subtype of T-ALL. A high MRD response rate was achieved by initial treatment with an impressive activity, particularly of induction II. Thymic T-ALL pts with MRD-IMR had a poorer OS; identification of this subgroup by sensitive MRD testing is important. T-LBL pts have similar outcomes as T-ALL. Nela/Cyclo consolidation was well tolerated but it remains open to which extent the compound contributed to the excellent OS. The most problematic subgroup is early T-ALL (43% estimated to be ETP) with poorer MRD response to standard chemotherapy, poor response to Nela in MolFail, and relevant relapse rate even after SCT. Further biological characterization and new treatment approaches are urgently needed for early T-ALL. Furthermore, additional MRD assessment by flow cytometry is important due to the frequent lack of clonal IG/TCR rearrangements in this subtype and MRD assessments should regularly continue after SCT to identify molecular relapses.
The trial was funded by Deutsche Krebshilfe; Nelarabin and financial support for documentation was provided by Novartis.
Disclosures
Goekbuget:Jazz Pharmaceuticals: Other: Research funding (institution); advisory board; MorphoSys: Other: Advisory board; Clinigen: Other: Advisory board; Incyte: Other: Research funding (institution); advisory board; Amgen: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Servier: Consultancy, Other: Research funding (institution); invited speaker; advisory board; Novartis: Other: Research funding (institution); advisory board; AbbVie: Other: Research funding (institution); AstraZeneca: Other: Invited talk for company-sponsored symposia (with honoraria); Cellestia: Other: Advisory board; Erytech: Other: Advisory board; Gilead/Kite: Other: Research funding (institution); invited talk; advisory board; Pfizer: Consultancy, Other: Research funding (institution); invited speaker; advisory board. Alakel:Pfizer: Consultancy, Honoraria. Topp:Amgen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Celgene: Consultancy; Roche: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; MacroGenics: Research Funding. Steffen:Jazz Pharmaceuticals: Other: Travel/Congress Participation Support; AbbVie: Other: Travel/Congress Participation Support. Wäsch:Pfizer: Consultancy, Honoraria. Viardot:Kite Gilead: Consultancy, Honoraria, Other: Support for meeting attendance; Novartis: Consultancy, Honoraria, Other: Support for meeting attendance; BMS: Consultancy, Honoraria, Other: Support for meeting attendance; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Other: Support for meeting attendance; Janssen-Cilag: Honoraria, Other: Support for meeting attendance; Astra Zeneca: Honoraria, Other: Support for meeting attendance; Incyte: Consultancy, Other: Support for meeting attendance. Nachtkamp:Jazz: Honoraria; BSH medical: Honoraria. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Fransecky:Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau. Buecklein:Novartis: Honoraria, Research Funding, Speakers Bureau; Pfizer: Research Funding, Speakers Bureau; Gilead: Honoraria; Miltenyi Biotech: Research Funding; Amgen: Honoraria. Schwartz:Amgen: Honoraria, Other: Advisory Board; CSI GmbH (Amgen/Jazz Pharmaceuticals): Speakers Bureau.
OffLabel Disclosure:
Nelarabin in First-Line
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal